Hematocure at the American Society of Clinical Oncology (ASCO) Annual Meeting
- Jun 8, 2025
- 9 min read

June 5, 2025.
The ASCO25 meeting that wrapped up earlier this week in Chicago was the largest ASCO Annual Meeting ever with just over 44,000 attendees. ASCO25 also saw one of its largest Hematology contingents, with slightly under 800 heme orals and posters presented. Hematocure’s team attended the meeting and summarized some of ASCO25 most important presentations in the field of malignant hematology.
Plasma cells neoplasia
Anti-BCMA CART CARVYKTI shone brightly with the unveiling by Peter Voorhees, MD of long-term follow-up data from the CARTITUDE-1 study. At the 5-Year mark, one third of the Relapsed Refractory (R/R) Multiple Myeloma (MM) patients were still in complete remission with no detectable molecular traces of disease. It is important to remember that CARTITUDE-1 enrolled a heavily pre-treated population of RRMM patients who at the time of study initiation had an expectation of no better than 6 months of Progression-Free Survival (PFS).
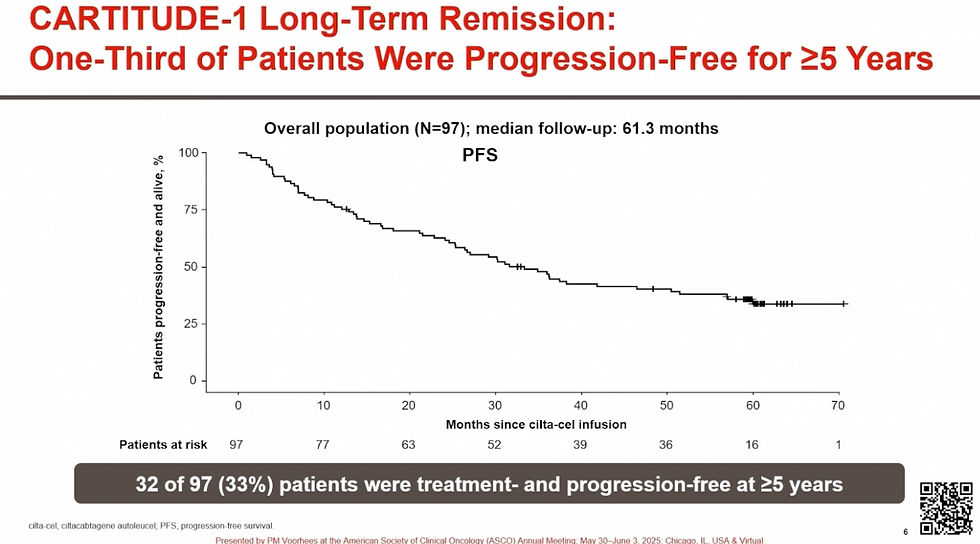
During the following experts panel session, Krina K. Patel, MD described the results as the “first potential functional cure for modern day relapsed refractory Multiple Myeloma”. The work was simultaneously published in the Journal of Clinical Oncology and highlighted in the New York Times.
Anti-CD38-containing quadruplets continue to strengthen their position as the Standard of Care for Newly Diagnosed Multiple Myeloma (NDMM). Multiple randomized frontline studies comparing anti-CD38 containing quadruplets to triplets were presented at ASCO25 and the updates, not surprisingly, emphasized Minimal Residual Disease (MRD) data given last year’s FDA decision on MRD-negative complete response as an acceptable early endpoint for accelerated approval in MM trials:
In PERSEUS (Darzalex- Velcade-Revlimid dexamethasone, or DVRd, versus Velcade-Revlimid dexamethasone, or VRd) updated by Philippe Moreau, MD , the rates of relapse or progression within 18 months of treatment (functional high-risk) were halved with DVRd versus VRd. Rates of sustained MRD negativity (10-5) ≥CR at 12 and 24 months were higher with DVRd (64.8% and 55.8%, respectively) versus VRd (29.7% and 22.6%). Sustained MRD-negativity was associated with a PFS benefit, with >95% achieving MRD negativity at 12 or 24 months remaining progression-free at 48 months.
Francesca Gay, MD, PhD updated the IsKia study (isatuximab-Kyprolis-Revlimid-dexamethasone, or Isa-KRd, versus Kyprolis-Revlimid-dexamethasone, or KRd) with a focus on the light consolidation portion of the study. Isa-KRd significantly increased the rate of MRD negativity (10-6) versus KRd post-light consolidation (74% Isa-KRd versus 64% with KRd). The benefits are seen even in the highest-risk patients. Isa-KRd light consolidation was well tolerated.
ADVANCE was presented by Ola C Landgren, MD, PhD . The trial enrolled NDMM patients independently of their transplant eligibility status. Subjects were randomized to DKRd or KRd for 8 cycles after which they were transplanted if MRD positive, whereas transplant was deferred if they were MRD negative. MRD negativity rates (10-5) at landmark was 59% with DKRd and 33% for KRd. At 31.2 months median follow-up, 92% were progression-free with DKRd versus 83% with KRd.
Aurore Perrot MD, PhD updated the MIDAS study, an ongoing Phase 3 trial evaluating MRD-guided consolidation strategy after Isa-KRd induction. In MRD-negative patients following Isa-KRd induction, Autologous Stem Cell Transplant (ASCT) consolidation did not improve MRD negativity rate compared to continuing Isa-KRD. In MRD-positive patients, tandem ASCT did not provide additional benefits in terms of MRD negativity compared to single ASCT. This work was simultaneously published in the New England Journal of Medicine.
New T-cell engagers on the horizon for MM
Early data on JNJ-79635322 (JNJ-5322), a novel BCMAxGPRC5DxCD3 trispecific was presented by Niels van de Donk, MD, PhD. In the First-In-Human study in RRMM, JNJ-5322 showed an Overall Response Rate (ORR) comparable to CARTs; JNJ-5322 ORR at the RP2D is 100% (≥CR of 70.4%) in BCMA/GPRC5D naïve patients.

JNJ-5322 appears to have an improved safety profile compared with the approved agents targeting BCMA or GPRC5D. Of note, the dysgeusia typically observed with TALVEY was minimal with JNJ-5322. It will be interesting to see the durability of the responses and the Overall Survival (OS) as the data matures.
o Other next-generation T-cell engagers for MM being currently evaluated in clinical trials and presented at ASCO25 included:
1) ISB2001, a BCMAxCD38xCD3 trispecific. The First-in-Human data presented showed an ORR of 75% across all 8 doses tested and a 64% Very Good Partial Response (VGPR) or better rate.
2) BCMAxCD3 etentamig which has the potential to be Best-In-Class BCMA bispecific with its bivalent BCMA binding moiety (only MM T-Cell Engager with such feature), a low activating CD3 domain designed reduce CRS and minimize T-cell exhaustion, and silenced Fc tail to allow for a longer half-life (thus the Q4W dosing). The two Phase 1/1b reported showed one the lowest CRS rate of any bispecific (30% with no Grade 3), and a 63-72% ORR across various risk groups. The Phase 3 Cervino study is ongoing.
NEXICART-2, the first US trial of a CART for R/R AL Amyloidosis, an unmet medical need, was presented by Heather J. Landau, MD. Data on NXC-201 was reported for 10 subjects. The product was safe with only low-grade CRS and no neurotoxicity observed. All patients (100%) experienced a rapid and deep hematological response. At Day 25, 8/9 evaluable patients were MRD negative. Beneficial organ responses were documented in 4/5 evaluable patients. No progression observed yet at a median follow-up of 121 days (range: 29-289). The study is ongoing with an additional 25 subjects planned to be enrolled.
Myeloid Malignancies
Despite recent progress, the treatment of AML, MDS and MPNs remains challenging, with median survival in the aggressive subtypes measured in months. Venetoclax resistance is also problematic. There are, however, novel therapeutic approaches on the horizon bringing new hope:
o Menin inhibitors. Revuforj (revumenib) was the first agent in this class approved last year. At this ASCO25, results for menin inhibitor ziftomenib from the KOMET-001 study were presented by Eunice S. Wang, MD. At the Recommeded Phase 2 Dose (RP2D) of 600 mg QD, ziftomenib showed a CR/CRh rate of 25% for the pooled Phase 1b/2 data, on par with that of revumenib from the AUGMENT-101 study that led to its approval. Ziftomenib was well tolerated with low rates of drug-related myelosuppression, no clinically significant QTc prolongation and, importantly, only mild and rare occurrences of Differentiation Syndrome (13%, Grade 3 only).
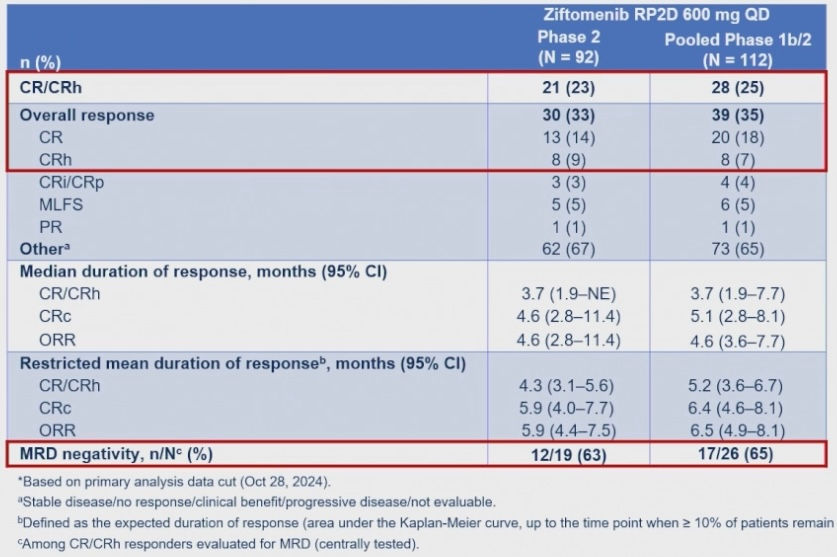
As the data was being disclosed at ASCO25, Kura Oncology and Kyowa Kirin announced that the FDA had granted Priority Review for ziftomenib in NPM1-mutant R/R AML.
This is a crowded field as there are currently six different menin inhibitors being evaluated in clinical trials. Aside from revumenib and ziftomenib, DSP-5336, Bleximenib (JNJ-7526617), DS-1594, and BMF-219 are at various stages of development.
o Next-generation Bcl-2 inhibitors. Phase 1b/2 study of lisaftoclax (APG-2575) combined with azacitidine in patients with treatment-naïve or prior venetoclax exposed myeloid malignancies was updated at ASCO25. Even though the numbers are small, responses to lisaftoclax in the 30-50% ORR range were seen in patients with R/R AML and High-Risk MDS, even in individuals refractory to venetoclax. Lisaftoclax is currently also undergoing Phase 3 clinical trials, including a global trial evaluating its efficacy and safety in combination in CLL/SLL patients who have previously been treated with a BTK inhibitor.
o Novel Immune Modulating approaches. The results from the Phase 1/2 BEXMAB study assessing the efficacy of macrophage checkpoint Clever-1 inhibition with bexmarilimab plus azacitidine in MDS were presented by Nadal Daver, MD . Most interesting were the responses seen in R/R MDS where the disease has often become resistant to azacitidine and other chemotherapeutic approaches. An ORR of 80% (65% per IWG2023) was observed in the twenty R/R MDS patients and the median OS estimate was 13.4 months.
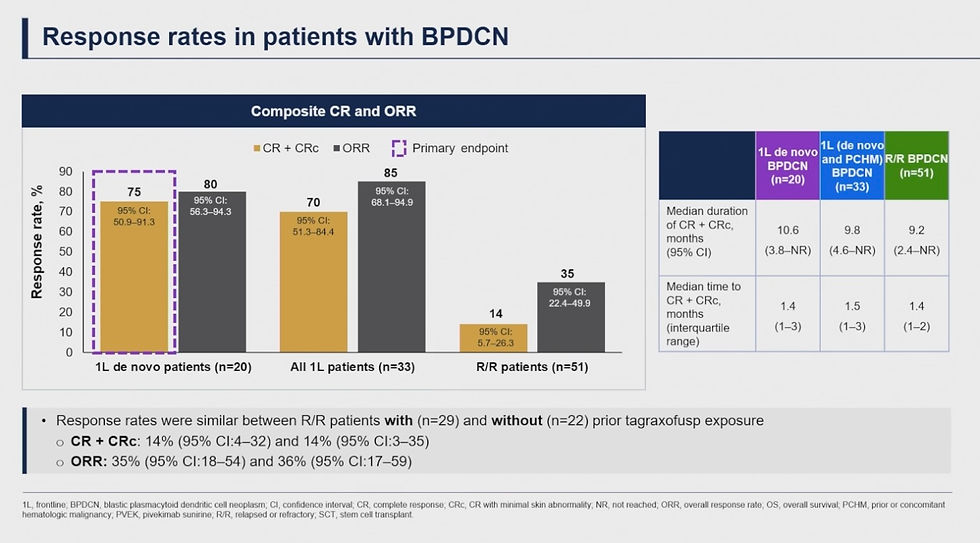
Naveen Pemmaraju, MD presented results from the Phase I/II CADENZA study assessing first-in-class CD123-Antibody-Drug Conjugate (CD123-ADC) pivekimab sunirine (PVEK) in patients with Blastic Plasmacytoid Dendric Cell Neoplasm (BPDCN), a rare and aggressive blood cancer. PVEK as a front-line treatment in newly diagnosed BPDCN was found to be safe and effective with a high ORR of 85%, a complete response rate of 70%, and a median OS of 16.6 months.
Lymphoid Malignancies
Next-generation Bruton’s Tyrosine Kinase (BTK) inhibitors in CLL/SLL were highlighted at ASCO25 with zanubrutinib leading the charge with readouts from various arms of its SEQUOIA study.
o SEQUOIA Arm C, updated by Constantine Si Lun Tam, MD, FRACP, FRCPA, MBBS reported on the largest prospective cohort of uniformly treated patients with del(17p) Treatment-Naïve (TN) CLL/SLL. With a median follow-up of 5 years, zanubrutinib demonstrates durable robust activity in del(17p) patients with an estimated 60-month PFS of 72.2%, similar to that observed in patients without del(17p) and highlighting that zanubrutinib overcomes the negative prognostic impact of del(17p). This data is in line with the results from the Phase 3 ALPINE study.
o Mazyar Shadman, MD, MPH presented the results of SEQUOIA Arm D investigating the combination of zanubrutinib + venetoclax in TN CLL/SLL patients. In patients without del(17p) and TP53 mutations, the 24-month PFS was 89% whereas in patients with del(17p) and/or TP53 mutations, the 24-month PFS was 94% and maintained at 36-month (88%). The best undetectable MRD (uMRD) in the peripheral blood was 59% regardless of mutational status. The combination was tolerated with no unexpected safety signal. This data highlights the potential for an all-oral, time limited zanubrutinib +venetoclax combination to drive disease control in CLL/SLL regardless of del(17p) and/or TP53 status.

SEQUOIA Arm D results were simultaneously published in the Journal of Clinical Oncology .
BTK inhibition or degradation is an active field of clinical development, with a number of early assets presented at ASCO25 including 4th generation HBW-3220, a reversible BTK inhibitor effective against BTK wild-type, C481S, L528W, and T474I mutations, as well as non-covalent LYN/BTK dual inhibitor DZD8586 that showed activity in CLL/SLL patients after covalent or non-covalent BTK inhibitors and BTK degraders.
CD19- and CD20-targeting approaches in lymphomas.
o With multiple agents approved in this class over the last few years (CD19xCD3 BLINCYTO; CD19 CARTs KYMRIAH, YESCARTA, and BREYANZI; CD20xCD3 LUNSUMIO, COLUMVI and EPKINLY), and loss of antigen emerging as a major mechanism of resistance to these, multi-antigen targeting as a mean to maximize activity and counter resistance has become the new front. With this backdrop, the Phase I results for the novel CD19/CD20 dual-targeting CART KITE-363 in R/R B-Cell Lymphoma were presented at ASCO25 by Saurabh Dahlya, MD, FACP. KITE-363 showed a good safety profile and demonstrated high rates of response in highly refractory CAR-naïve in a Large B-Cell Lymphoma (LBCL) population, especially at Dose Level 3 of 2x106 CART cells: ORR of 87%, CR rate of 76%, and a medium Duration of CR that has not been reached yet. Robust KITE-363 cell expansion was seen at Dose Level 3. Given these promising early results, KITE-363 is being further developed in LBCL, and being explored in autoimmune diseases.

o On May 23, the FDA Oncologic Drugs Advisory Committee (ODAC) voted 8-1 against the applicability of STARGLO trial results to U.S. patients with relapsed/refractory DLBCL. The confirmatory global Phase 3 STARGLO trial which assessed glofitamab (Glofit)-GemOx versus R-GemOx in R/R DLBCL was conducted during the COVID-19 pandemic and enrolled only 25 patients from the United States, or 9% of the trial population. This was an important warning to pharmaceutical sponsors running global clinical trials to include enough patients that reflect US medical practices or they could face increased regulatory risk when seeking approval from the FDA. At ASCO25, Jeremy S. Abramson, MD, presented the latest update from STARGLO. After 2 years of follow-up, Glofit-GemOx showed sustained benefit in survival and response outcome versus R-GemOx: median PFS with Glofit-GemOx was 13.8 months (versus 3.6 months with R-GemOx) with 46.5% of these patients being progression free at 18 months, and 54.4% still alive after 24 months. The safety profile remained manageable and consistent with known risk for each study drug. Immune recovery was observed at 18-24 months after fixed duration treatment of Glofit-GemOx. An expansion cohort is ongoing enrolling mostly US patients.
o Among the newest approaches presented at ASCO25 for LBCL were ADCs. Three such assets were discussed:
1) Philippe Armand, MD, PhD updated the waveLINE-003 Phase 2/3 study assessing Zilovertamab vedotin (ZV), a novel ROR1-ADC, in R/R DLBCL after ≥1 lines of therapy who were ineligible for CAR-T, ASCT, or failed these therapies. At the RP2D of 1.75 mg/kg, the ORR was 56% (8 CR, 1 PR), and median overall survival was not reached with 6-month OS rate 78.8%. The study is proceeding to the Phase 3 portion randomizing patients to ZV-RGemOx versus RGemOx.
2) Data was presented for a Chinese Phase 1b/2 study of CD79b-ADC SHR-A1912 in combination with R-GemOx in R/R DLBCL. Among the 37 patients dosed, 19 achieved a CR, and 8 a PR. The ORR was 73.0% and CR rate was 51.4%. Twenty-three of the 27 responders showed an objective response at their first anti-tumor assessment, with a time to response of 1.4 month. All responses were ongoing as of the cutoff date.
3) Dai Maruyama, MD, PhD from the Japanese Foundation for Cancer Research covered the Trial-In-Progress of a First-in-Human study of ABBV-291, another CD79b-ADC, in patients with R/R B-cell non-Hodgkin lymphoma.
We will hear more about many of these assets and studies at the EHA2025 Congress starting next week and will be following progress in the Hematology field throughout the year. Stay tuned!
Additional meeting notes available on request. Contact us at: info@hematocure.com



Comments